Hydrogen bonded ethanol molecules whose oxygen acts as proton donor... | Download Scientific Diagram

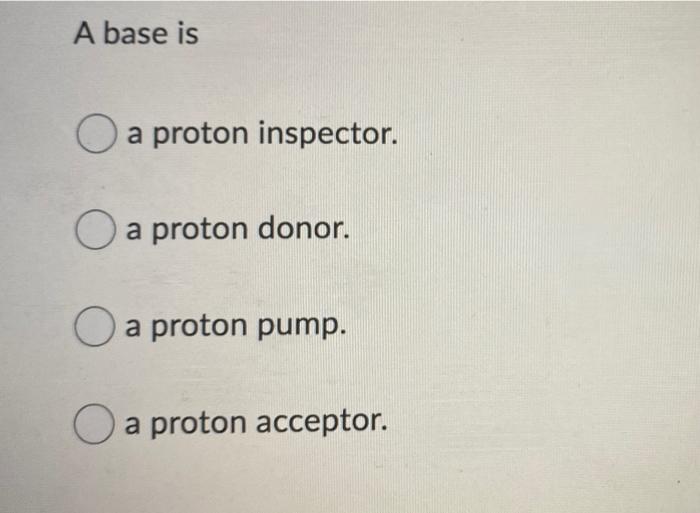
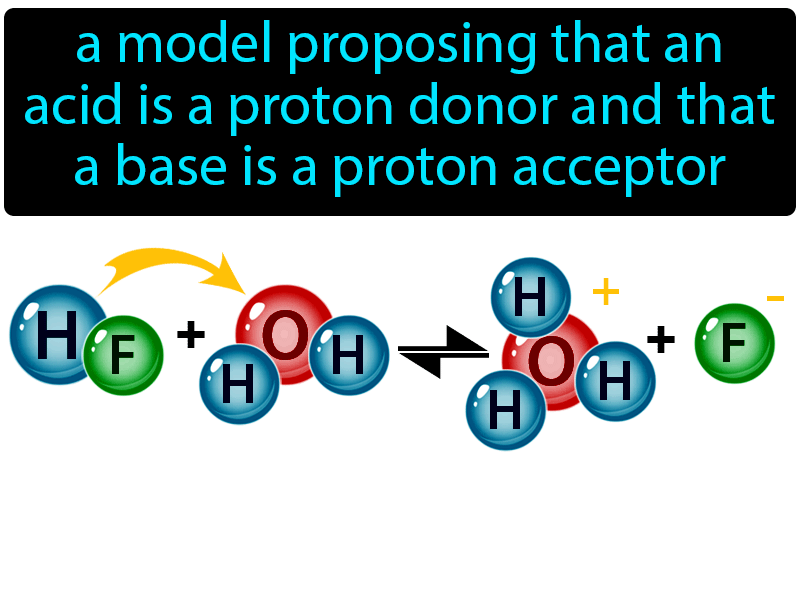
Acids and Bases. Acids & Bases The Bronsted-Lowry model defines an acid as a proton donor. A base is a proton acceptor. Note that this definition is based. - ppt download

Recap – Last Lecture An acid is a proton donor A base is a proton acceptor A conjugate pair differ by H + Strong A/B is completely dissociated Weak A/B. - ppt



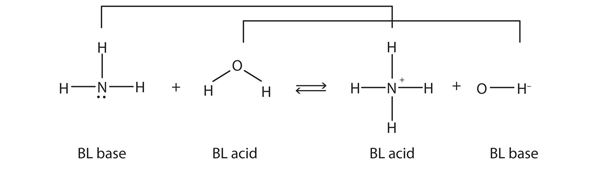


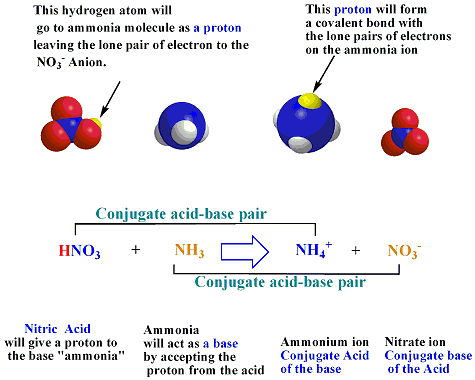

/chapter3/pages19and20/page19and20_files/abexample.png)