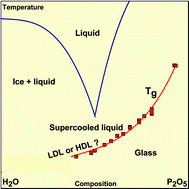
Heat capacity and glass transition in P2O5–H2O solutions: support for Mishima's conjecture on solvent water at low temperature - Physical Chemistry Chemical Physics (RSC Publishing)

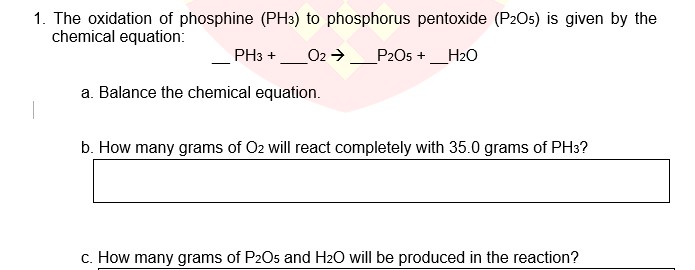
N2 (g) + 3H2 (g) 2NH3 (g) How many liters of ammonia (NH3) can be produced from 40 L of N2? What mass of N2 is required to produce 35 L

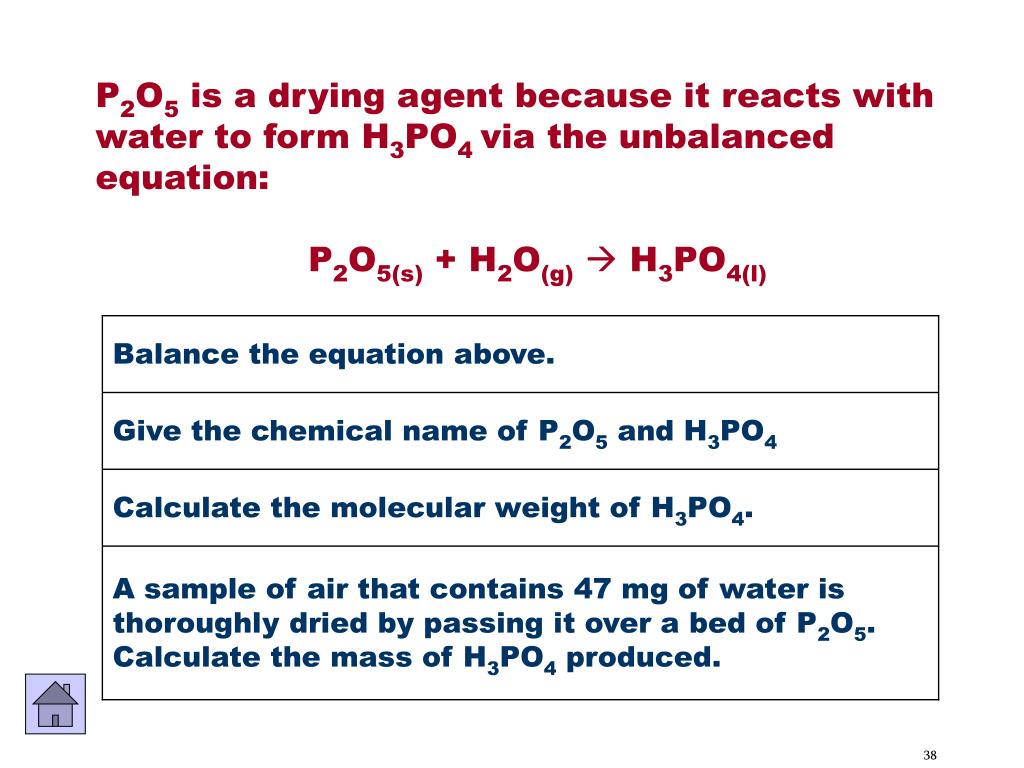
One mole of P2O5 undergoes hydrolysis as P2O5 + H2O⟶H3PO4 The normality of the phosphoric acid formed is (The volume of solution is 1 L)

The system Al2O3-P2O5-H2O at temperatures below 200 °C: Experimental data on the stability of variscite and metavariscite AlPO4·2H2O | Semantic Scholar

One mole of P2O5 undergoes hydrolysis as P2O5 + H2O⟶H3PO4 The normality of the phosphoric acid formed is (The volume of solution is 1 L)
ntP2O5 on treatment with exces s of H2O followed by excess of NH4OH forms (NH4)2HPO4. If hundred gram of (NH4)2HPO4 is formed then find out the mass of p 2 o5 initial

Total Ni, exchangeable ions, available P2O5, and pH (H2O) in the soils... | Download Scientific Diagram
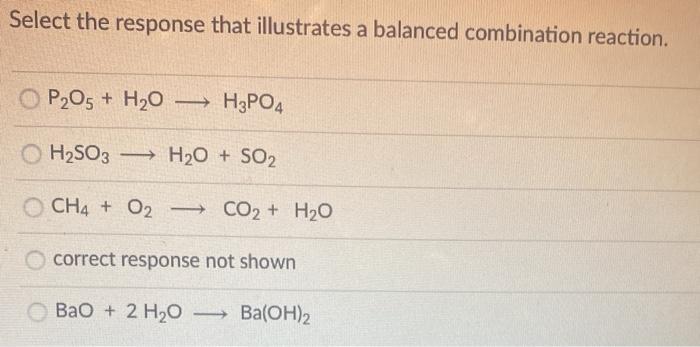
Complete and balance the following equation (i) P2O5 + H2O → ............ (ii) S + O2 → ................ - Sarthaks eConnect | Largest Online Education Community