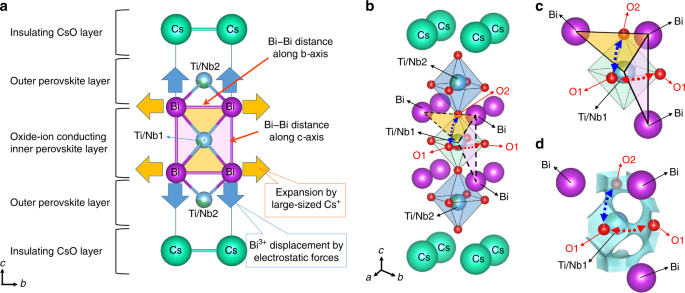
Associating High Oxide-Ion Conductivity and Conduction Mechanisms with Local Atomic Environments in Na0.5Bi0.5–xTi1–yMgyO3−δ | The Journal of Physical Chemistry C
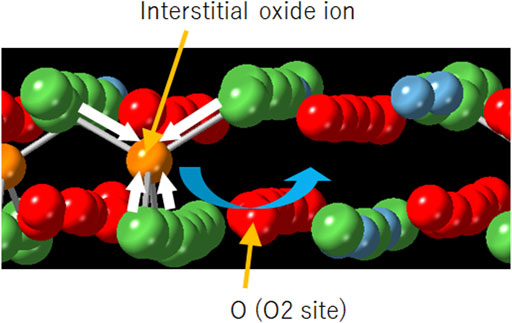
Frontiers | Effects of Ca substitution on the local structure and oxide–ion behavior of layered perovskite lanthanum nickelate

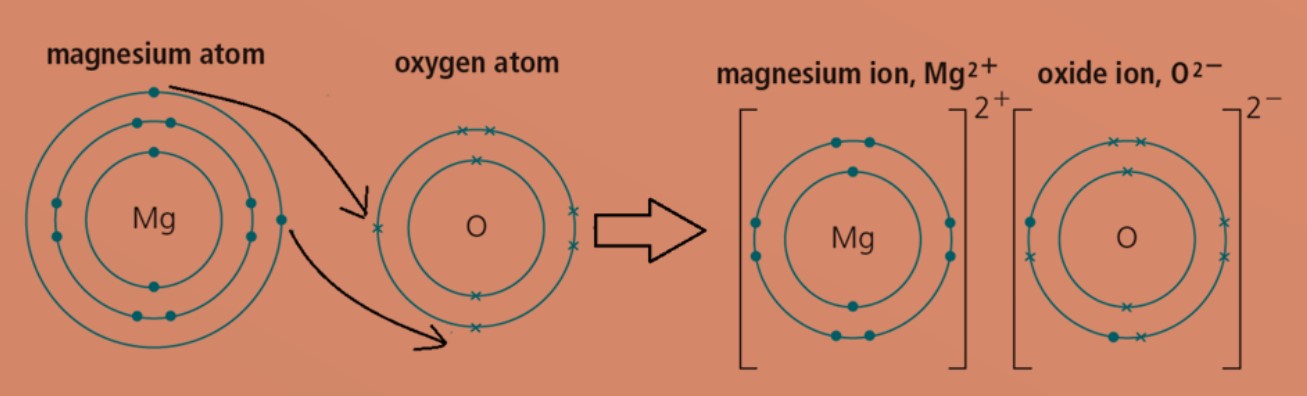
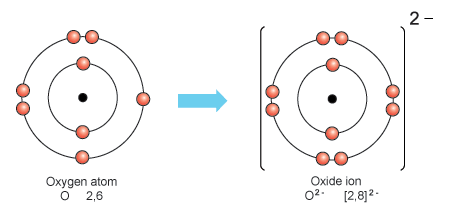
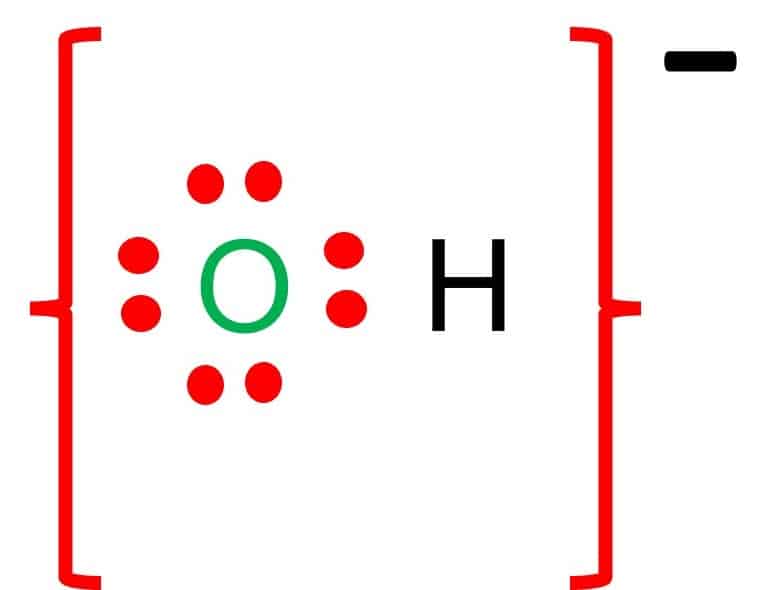
The formation of the oxide ion, O ^2 - (g) , from oxygen atom requires first an exothermic and then an endothermic step as shown below: O(g) + e^-→ O^-(g); Δ H^∘ = -

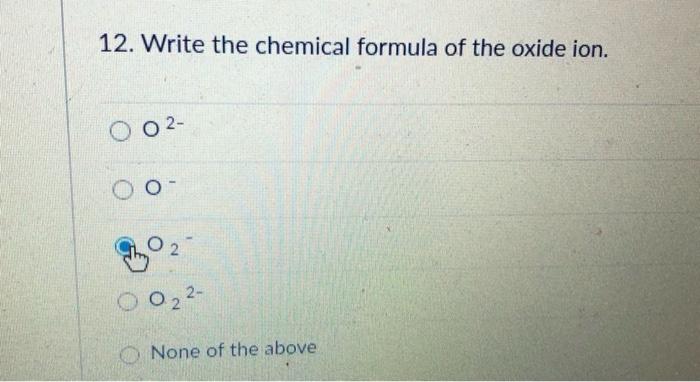
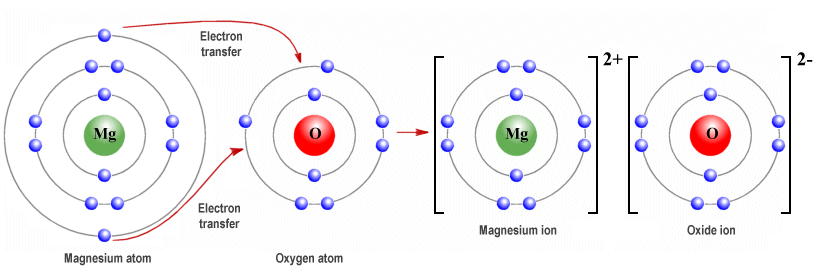
Chapter 20. Calculating Oxidation Numbers Each oxide ion has a charge of -2 7 oxide ions have a subtotal charge of -2 x 7 = -14 Since the formula has. - ppt download
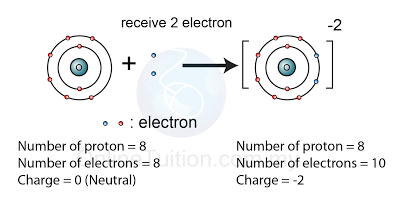
What is the difference between atoms and ions; and covalent compounds and ionic compounds? | Socratic