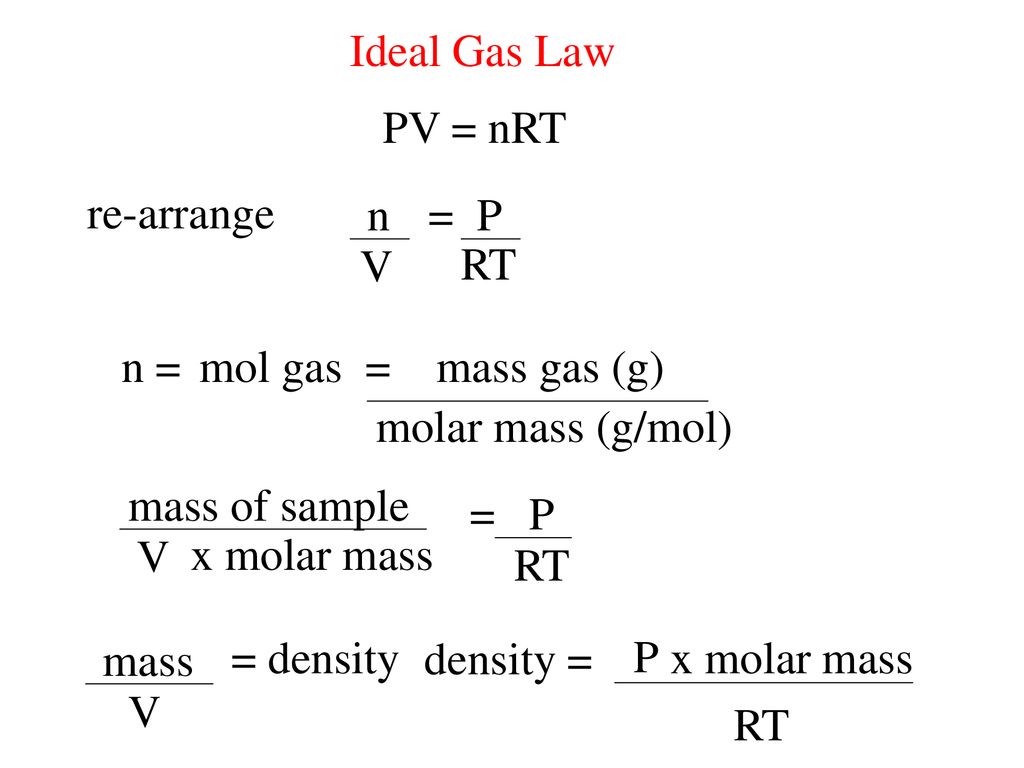
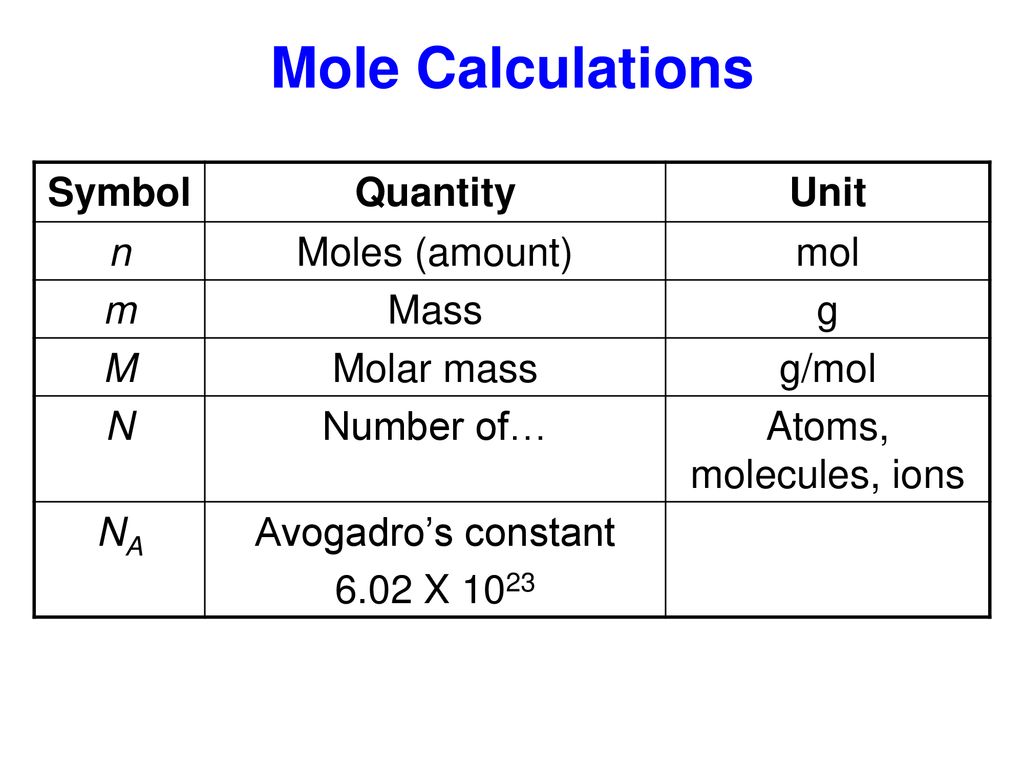
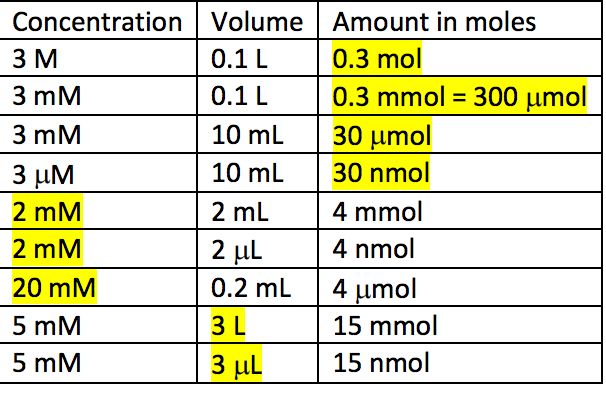
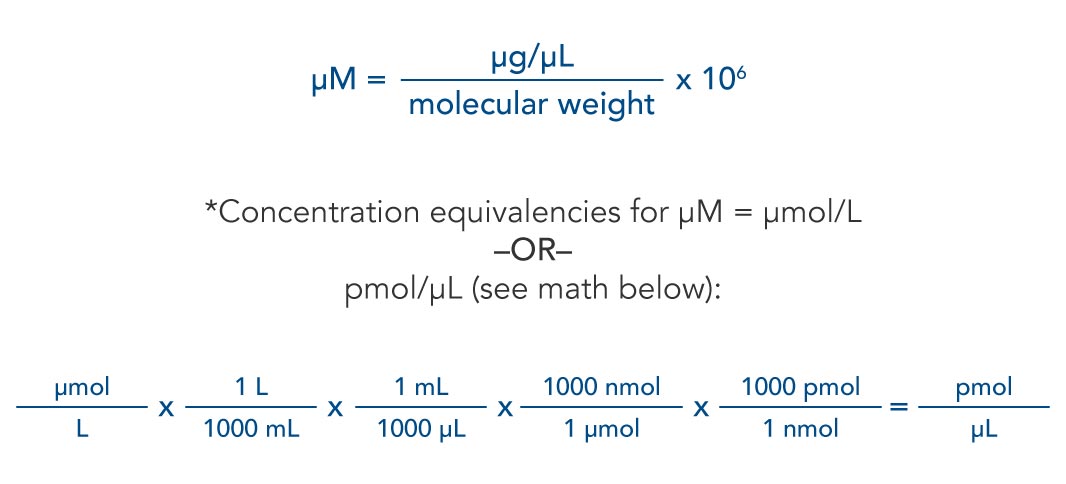
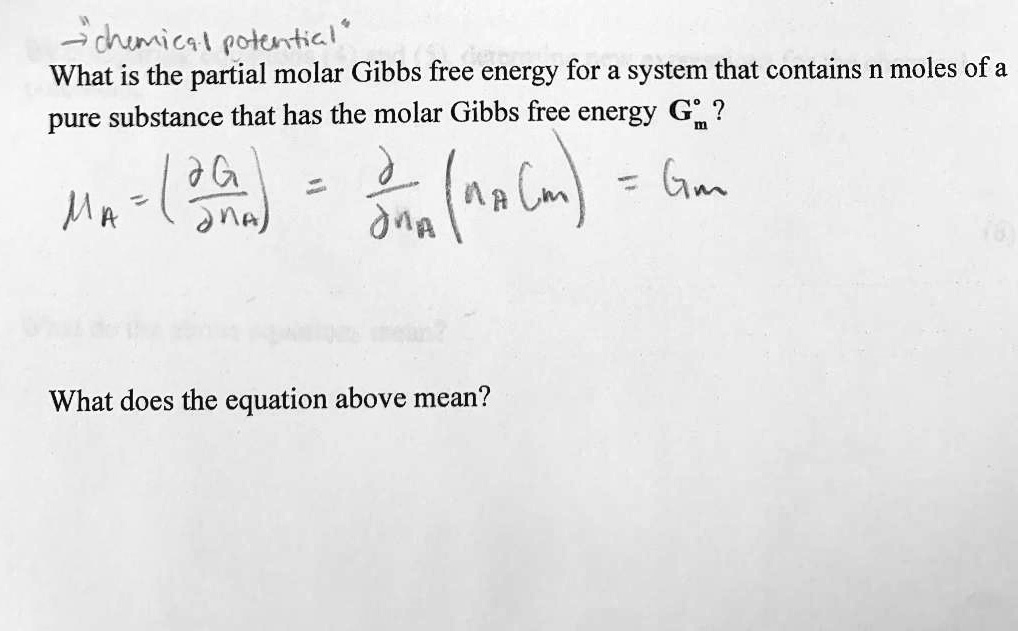= RT , at high pressure, the Van der Waals equation gets reduced to : Using Van der Waals equation, [ P + a/V^2 ](V - b) = RT , at high pressure, the Van der Waals equation gets reduced to :](https://dwes9vv9u0550.cloudfront.net/images/4070363/cebcd82b-7eb7-40b6-b09d-f4dcd3623226.jpg)
Using Van der Waals equation, [ P + a/V^2 ](V - b) = RT , at high pressure, the Van der Waals equation gets reduced to :
nta sample contain N moles of diatomic gas at temprature T . Molecules of gas get dissociated into atoms , temp remain constant. find change in internal energyn

An equilibrium mixture in a vessel of capacity 100 litre contains 1 mol N2 , 2 mol O2 and 3 mol NO . No. of moles of O2 to be added so