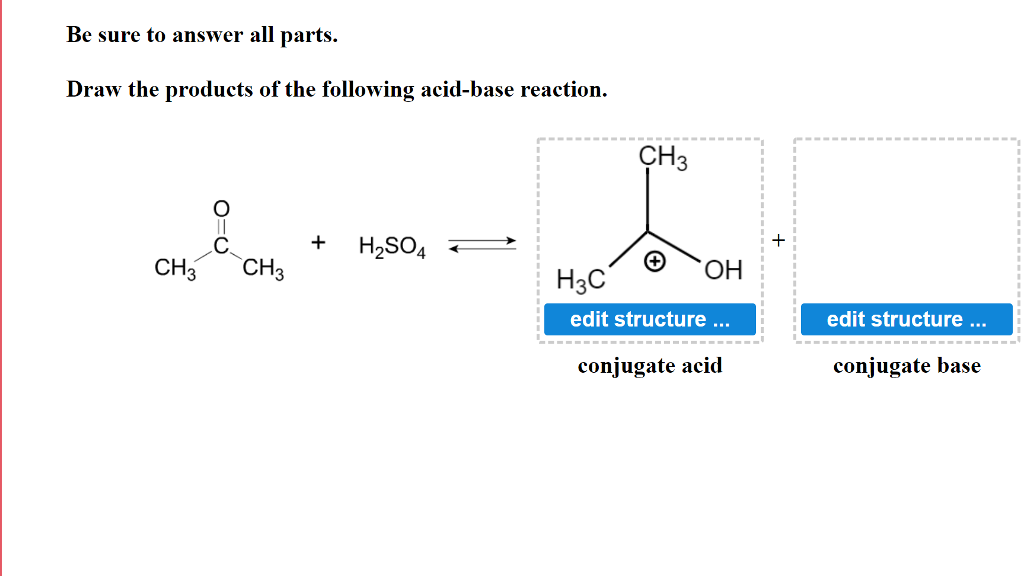
Use your understanding of molecular structure to explain why the conjugate bases of acids like formic acid CHOOH, acetic acid CH3COOH, and phosphoric acid are only stable enough to be weak acids;
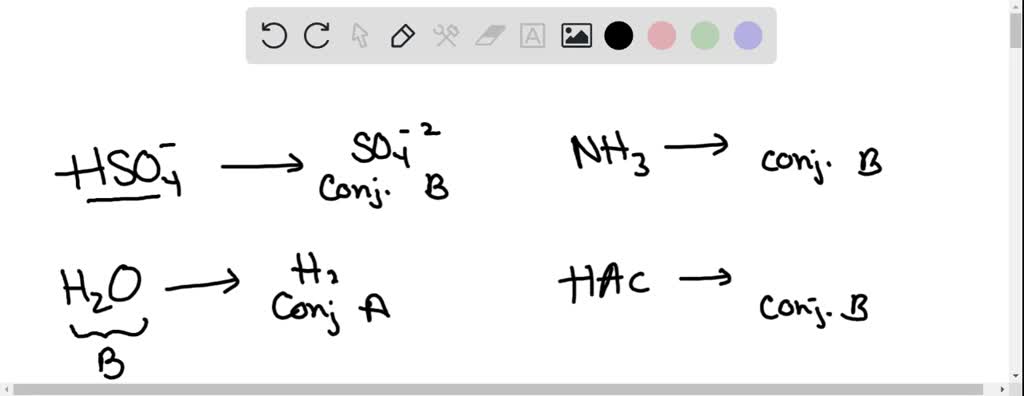
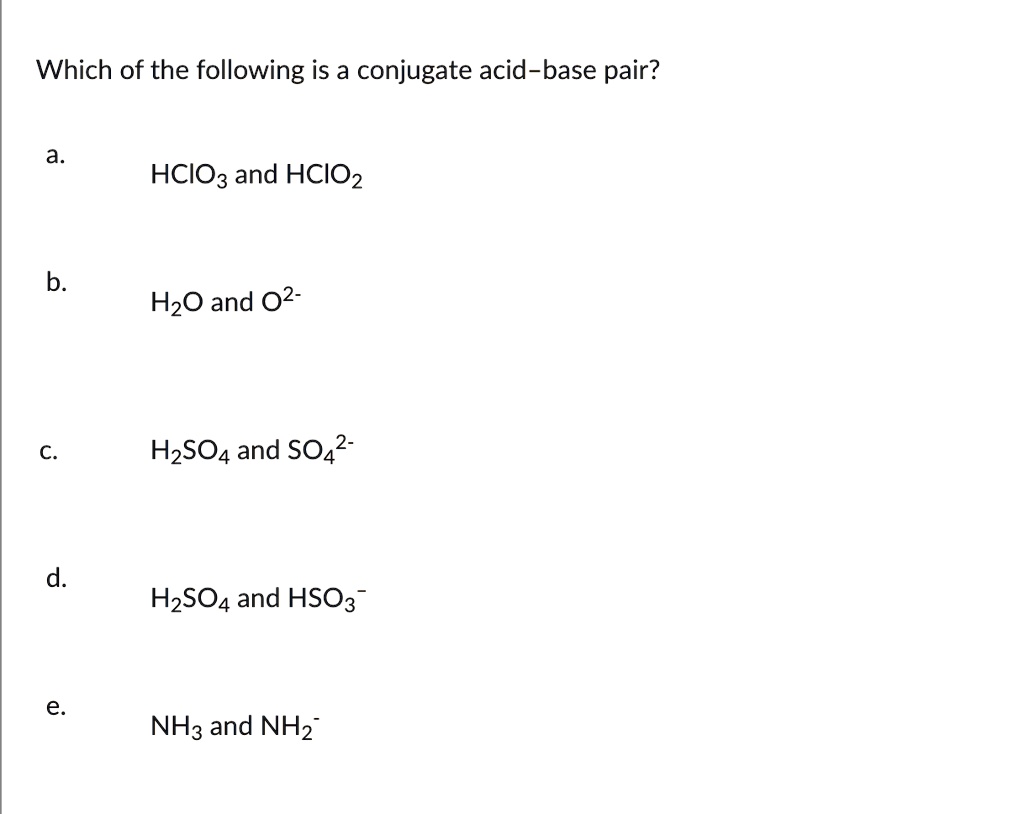
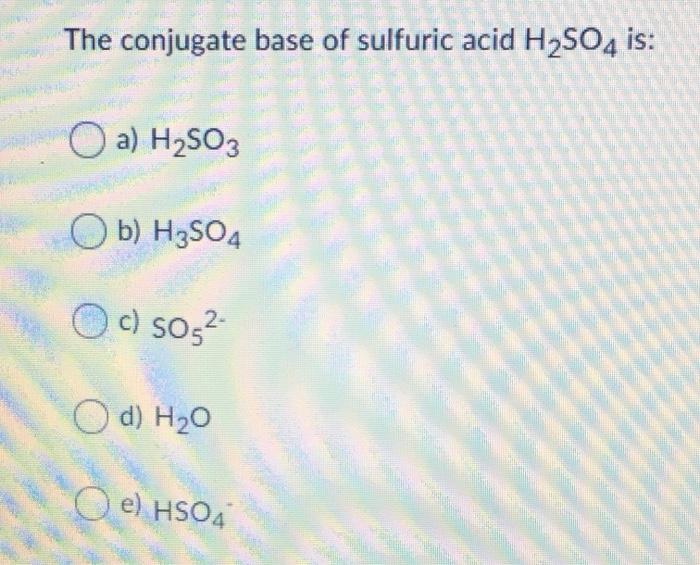
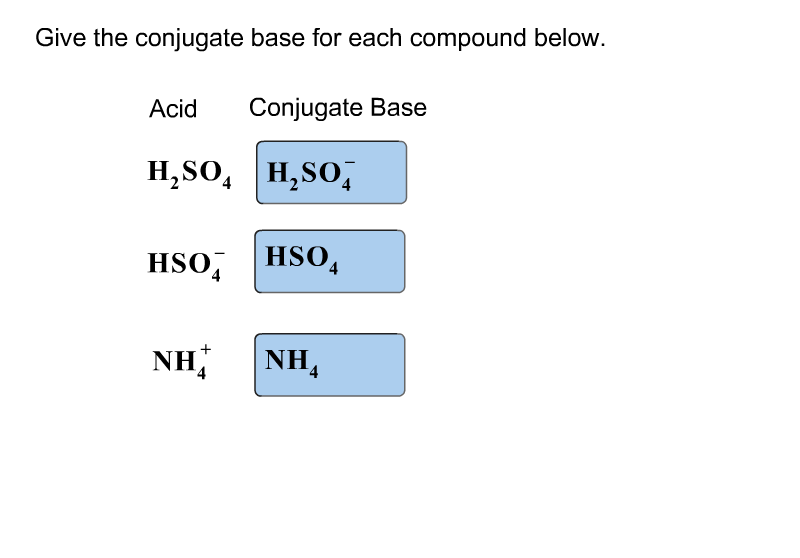
SOLVED: Which of the following is a CORRECTLY matched pair?Required to answer. Single choice. conjugate base of HSO4- is H2SO4 conjugate acid of H2O is OH- conjugate base of NH3 is NH4+
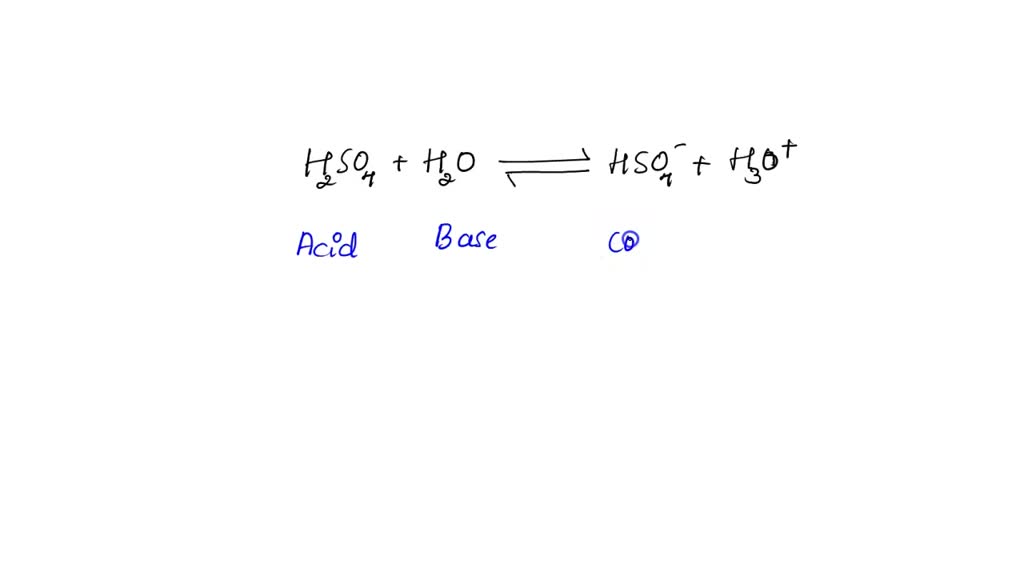
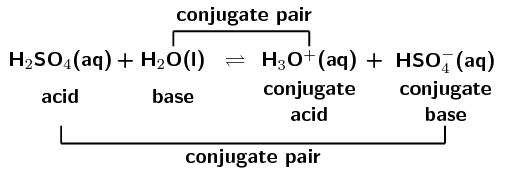
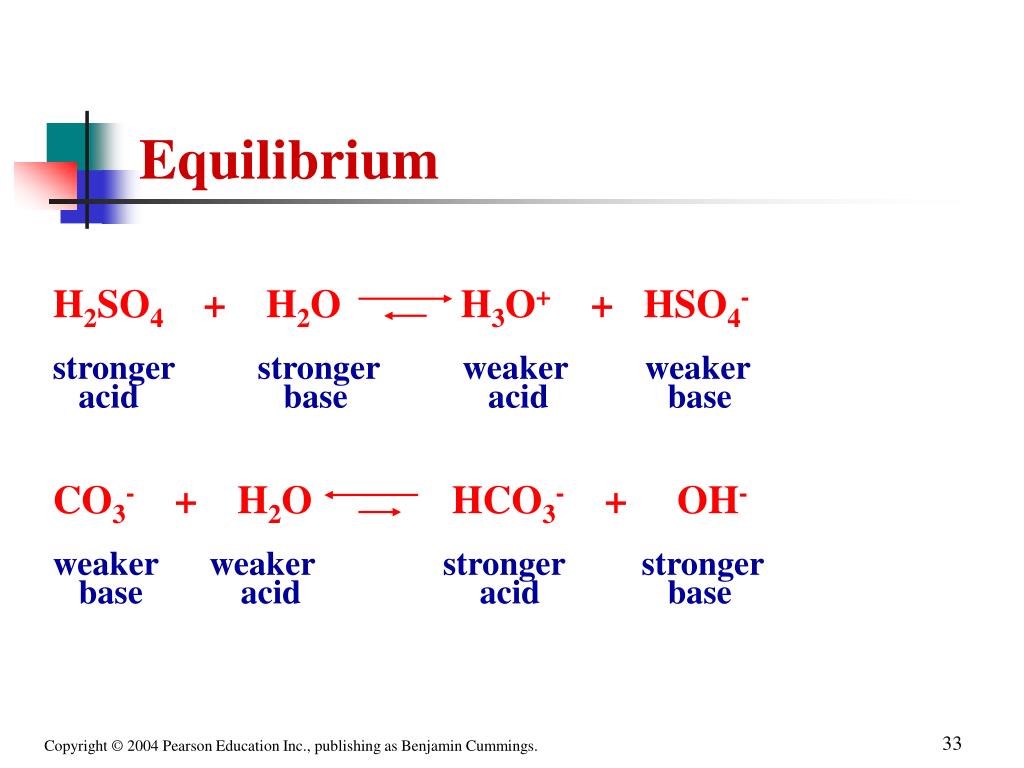
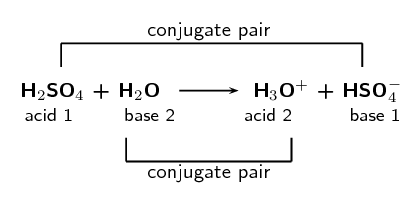
SOLVED: In the following equation, identify the Bronsted Lowry conjugate base H2SO4 + H2O <-> HSO4 - + H3O+ Group of answer choices H2SO4 HSO4- H2O H3O+

Ammonia Catalyzed Formation of Sulfuric Acid in Troposphere: The Curious Case of a Base Promoting Acid Rain | The Journal of Physical Chemistry A

organic chemistry - Why Does A Brønsted–Lowry Acid Accept Proton from Stronger Acid? - Chemistry Stack Exchange

Write a balanced equation for the dissociation of the following in water and identify the conjugate acid - base pairs. (i) NH4^(+) (ii) H2SO4 (iii) CH3COOH