Balance the following equations by oxidation number method 1. K2Cr2O7 + KI + H2SO2 → K2SO4 + Cr2(SO4)3 + I2 + H2O - Sarthaks eConnect | Largest Online Education Community
![Give the oxidation state, d - orbital occupation and coordination number of the central metal ion in the following complex. [Mn(H2O)6]SO4 . Give the oxidation state, d - orbital occupation and coordination number of the central metal ion in the following complex. [Mn(H2O)6]SO4 .](https://haygot.s3.amazonaws.com/questions/1873456_1575827_ans_3125df065a114c8e8610e9410b778e2a.jpeg)
Give the oxidation state, d - orbital occupation and coordination number of the central metal ion in the following complex. [Mn(H2O)6]SO4 .

Balance the reaction and explain what type of the reaction is: H_2SO_4 + B(OH)_3 to B_2(SO_4)_3+H_2O | Homework.Study.com
, RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry RE(SO4)[B(OH)4](H2O), RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.7b02317/asset/images/large/ic-2017-02317c_0008.jpeg)
RE(SO4)[B(OH)4](H2O), RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry

Solubility of FeSO4·7H2O in the H2SO4–Ti(SO4)2–H2O, H2SO4–MgSO4–H2O, and HCl–H2O Systems from 278 to 313 K | Journal of Chemical & Engineering Data

![Answered: a. CГОЗ HO H,SO4, H2O b. [1] O3 [2] H20… | bartleby Answered: a. CГОЗ HO H,SO4, H2O b. [1] O3 [2] H20… | bartleby](https://content.bartleby.com/qna-images/question/d66f1a49-6a74-4120-82f6-39c04434f2ae/0caae1aa-a18d-4475-b945-b3b8c68a132a/nw3bhka.png)




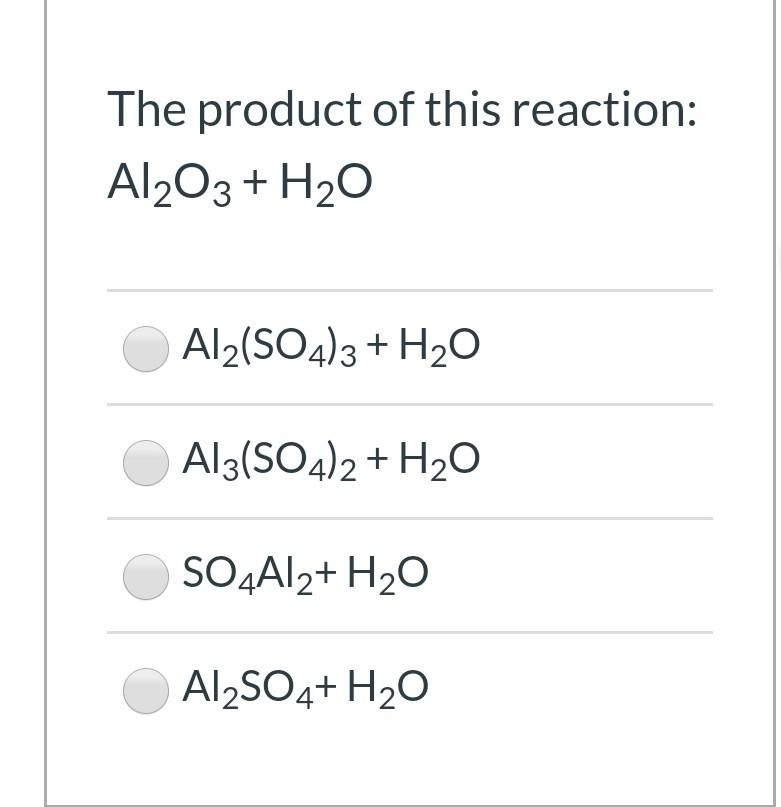




![Aluminium Sulfate Octahydrate [Al2(SO4)3.8H2O] Molecular Weight Calculation - Laboratory Notes Aluminium Sulfate Octahydrate [Al2(SO4)3.8H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/aluminium-sulfate-octahydrate-molecular-weight-calculation-300x178.jpg)