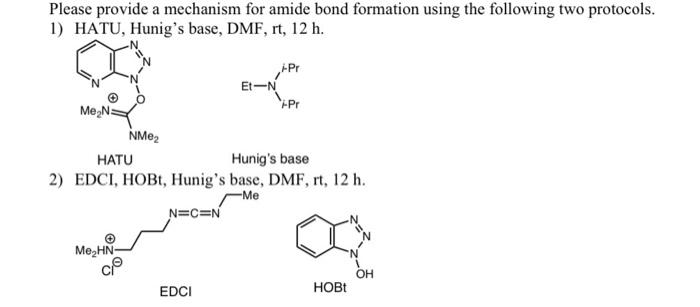
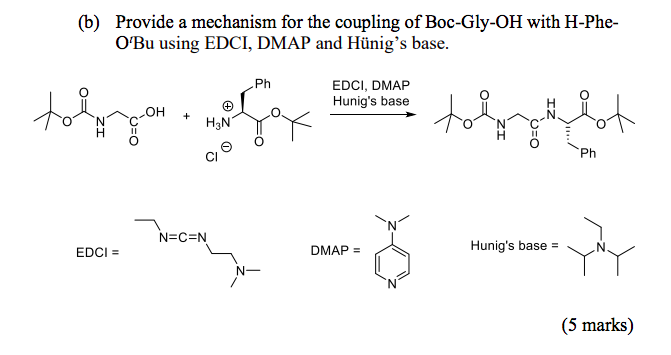
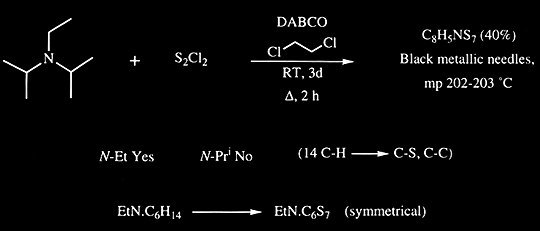
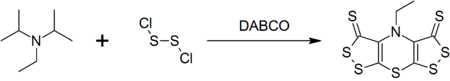Supporting Information Continuous-flow synthesis of primary amines: Metal-free reduction of aliphatic and aromatic nitro derivat

Hunig's base catalyzed synthesis of new 1-(2,3-dihydro-1H-inden-1-yl)-3-aryl urea/thiourea derivatives as potent antioxidants and 2HCK enzyme growth inhibitors - ScienceDirect
![PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3c974f939a32262aeed197ab08f1ab631c012982/4-Table2-1.png)
PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar
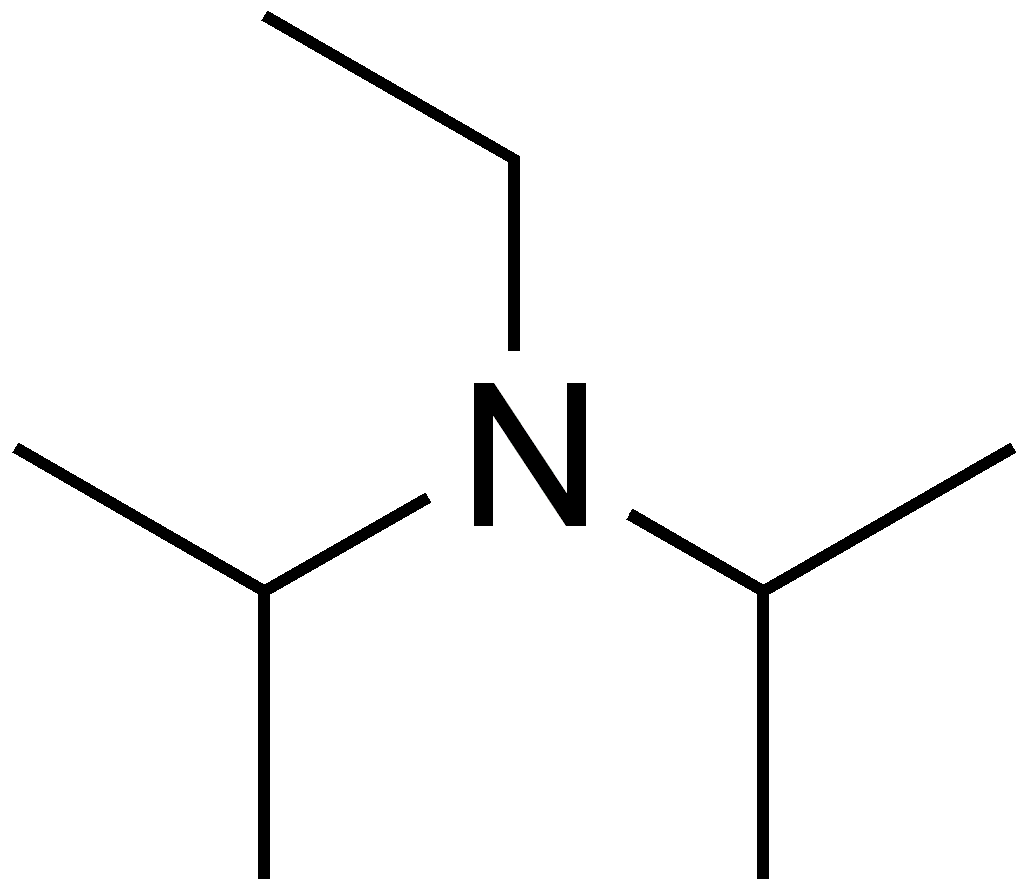
DIPEA (N,N-diisopropylethylamine, Hunig's base) molecule. Stylized skeletal formula (chemical structure). Atoms are shown as Stock Photo - Alamy
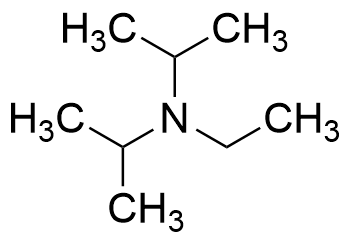
7087-68-5 | N,N-Diisopropylethylamine | 1,1'-Dimethyltriethylamine; Bis(1-methylethyl)ethylamine; DIEA; DIPEA; Diisopropylethylamine; Ethyl-N,N-diisopropylamine; Ethyldiisopropylamine; Huenig's base; Hunig's base; Hunig's reagent; N,N-Bis(1-methylethyl ...
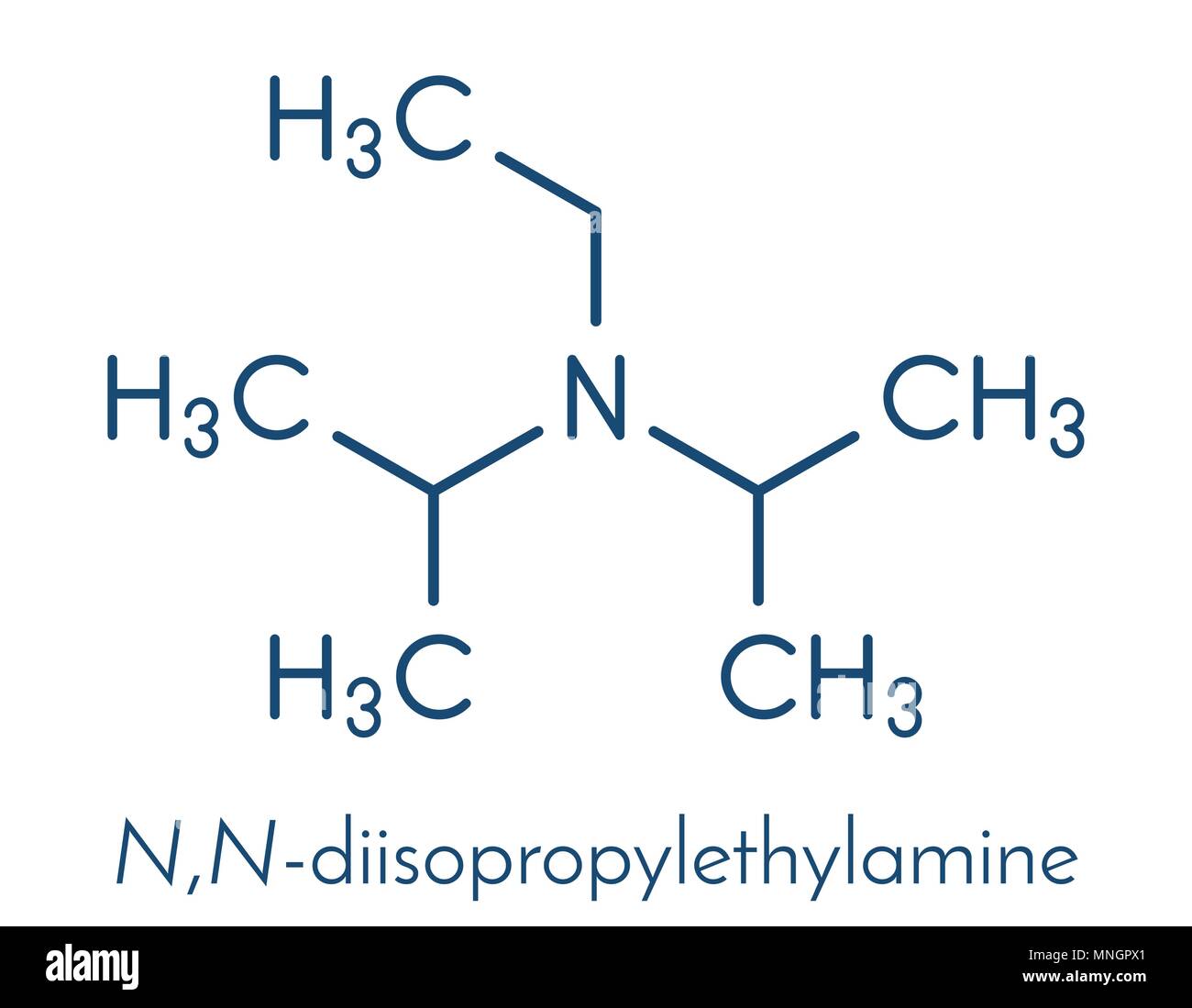
DIPEA (N,N-diisopropylethylamine, Hünig's base) molecule. Skeletal formula Stock Vector Image & Art - Alamy

Dipea Nndiisopropylethylamine Hunigs Base Molecule Skeletal Stock Vector (Royalty Free) 1093026992 | Shutterstock

A Homage to Siegfried Hünig and His Research - Reissig - 2021 - Angewandte Chemie International Edition - Wiley Online Library

Dipea Nndiisopropylethylamine Hunigs Base Molecule Skeletal Stock Vector (Royalty Free) 1093026992 | Shutterstock