Em relçao aos compostos ionicos: Fe2(co3) 3, Na2504 e k3p04. A) Indique os ions que formam: B) - Brainly.com.br
![SOLVED: Which is the CORRECT Ksp expression for Fe2(CO3)3? Ksp 0 [Fe]? [CO3]' [Fe3+j? [CO32-13 [Fest] [8O321 / [Fez(COs):] [Fe3+j? [CO32-1 / [Fez(CO3)3] [Fe]? [CO3]? [Fez(CO3)3] [Fes+] [CO3?-] SOLVED: Which is the CORRECT Ksp expression for Fe2(CO3)3? Ksp 0 [Fe]? [CO3]' [Fe3+j? [CO32-13 [Fest] [8O321 / [Fez(COs):] [Fe3+j? [CO32-1 / [Fez(CO3)3] [Fe]? [CO3]? [Fez(CO3)3] [Fes+] [CO3?-]](https://cdn.numerade.com/ask_images/6b9de619ef914826b94338cd7f5d81af.jpg)
SOLVED: Which is the CORRECT Ksp expression for Fe2(CO3)3? Ksp 0 [Fe]? [CO3]' [Fe3+j? [CO32-13 [Fest] [8O321 / [Fez(COs):] [Fe3+j? [CO32-1 / [Fez(CO3)3] [Fe]? [CO3]? [Fez(CO3)3] [Fes+] [CO3?-]

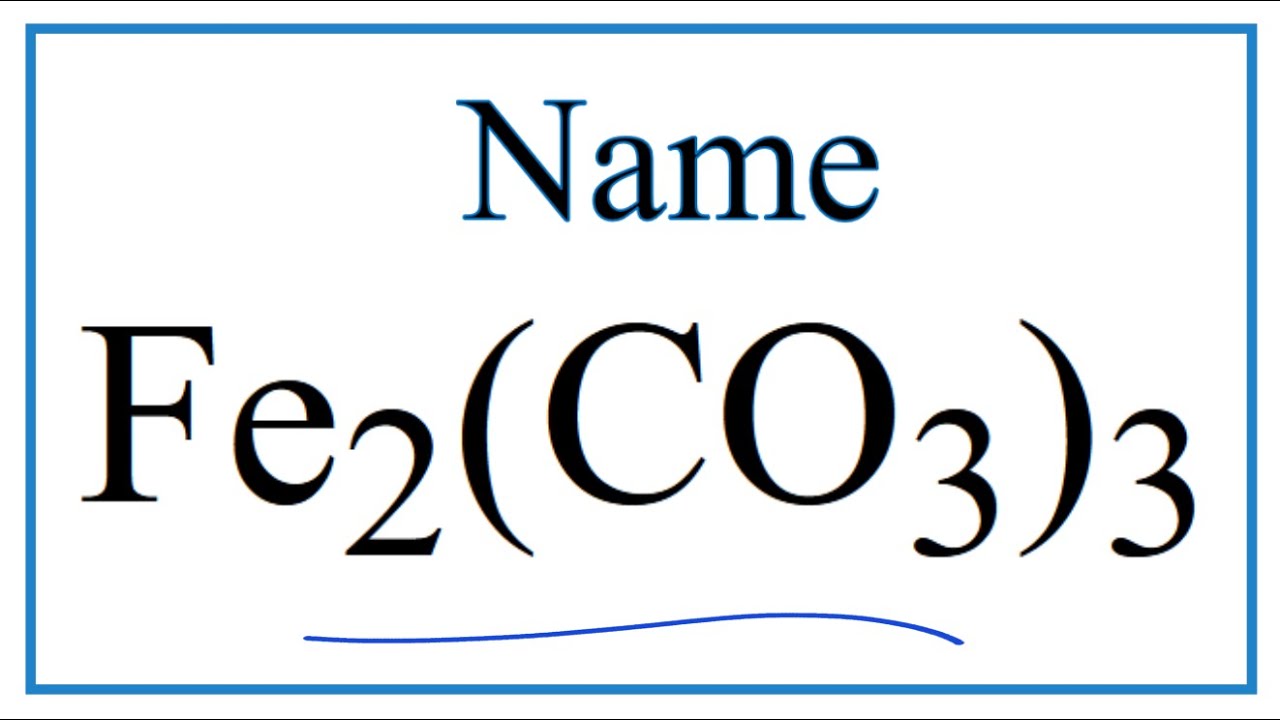
Oxidation Number for Fe2(CO3)3 . Oxidation state of fe2(co3)3 . Iron(III)carbonate oxidation state - YouTube

OneClass: 2. NaNO3+ PbO â†'Pb(NOJ2 + Na20 3. C2H4 + 02 4. Agl + Fe2(CO3)3 â†'Fels + Ag2co, 5. Al + HC...

Napisz równania reakcji otrzymywania Fe2(CO3)3 2 dowolnymi metodami. Opisz słownie przebieg reakcji. - Brainly.pl


_how-to-write-the-name-for-fe2co33.jpg)