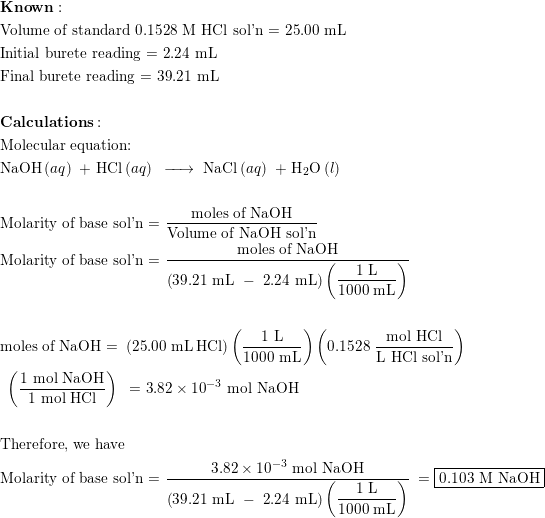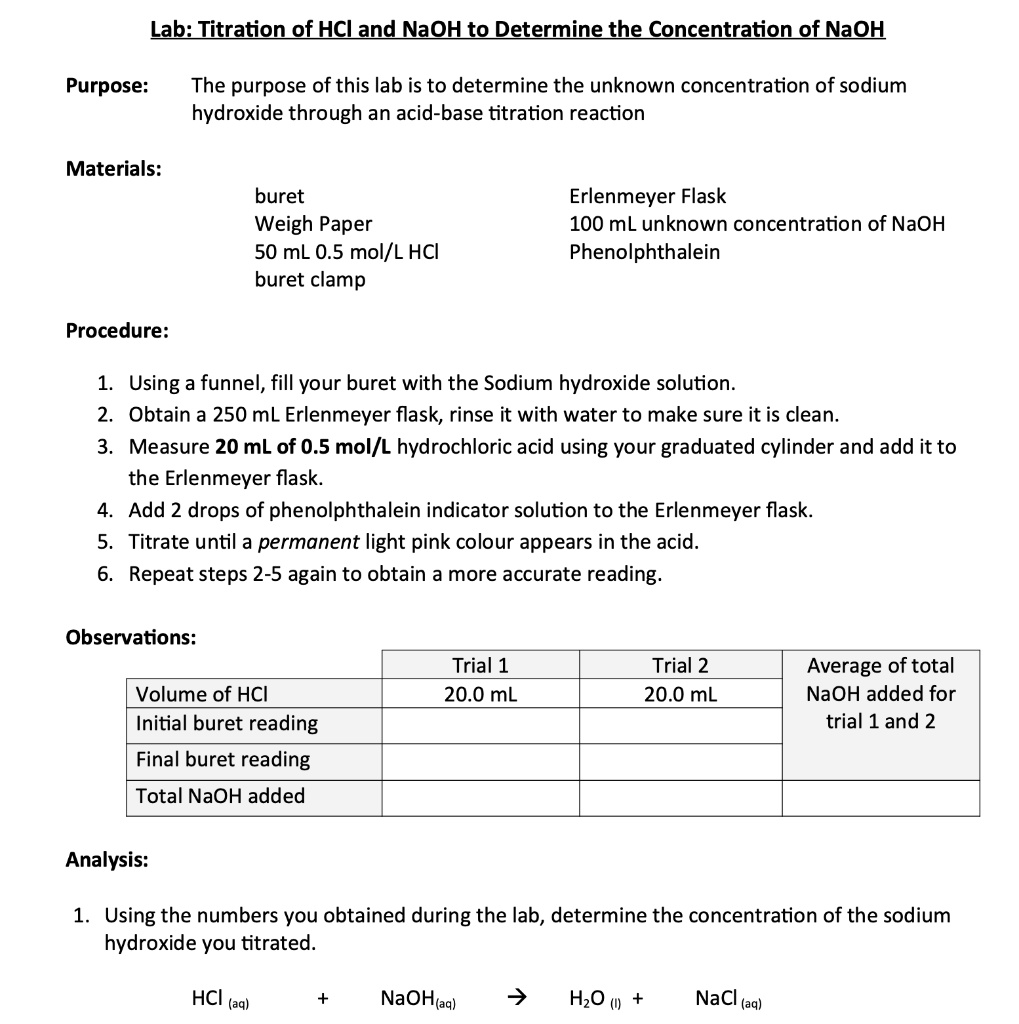
SOLVED: Lab: Titration of HCLand NaOHto Determine the Concentration of NaOH Purpose: The purpose of this lab is to determine the unknown concentration of sodium hydroxide through an acid-base titration reaction Materials:
Acids and bases, acid-base reaction, neutralization reaction, HCl, NaOH, NaCl, H2O, salt, water, chemical reaction, test setup, erlenmeyer, laboratory materials Stock ベクター | Adobe Stock

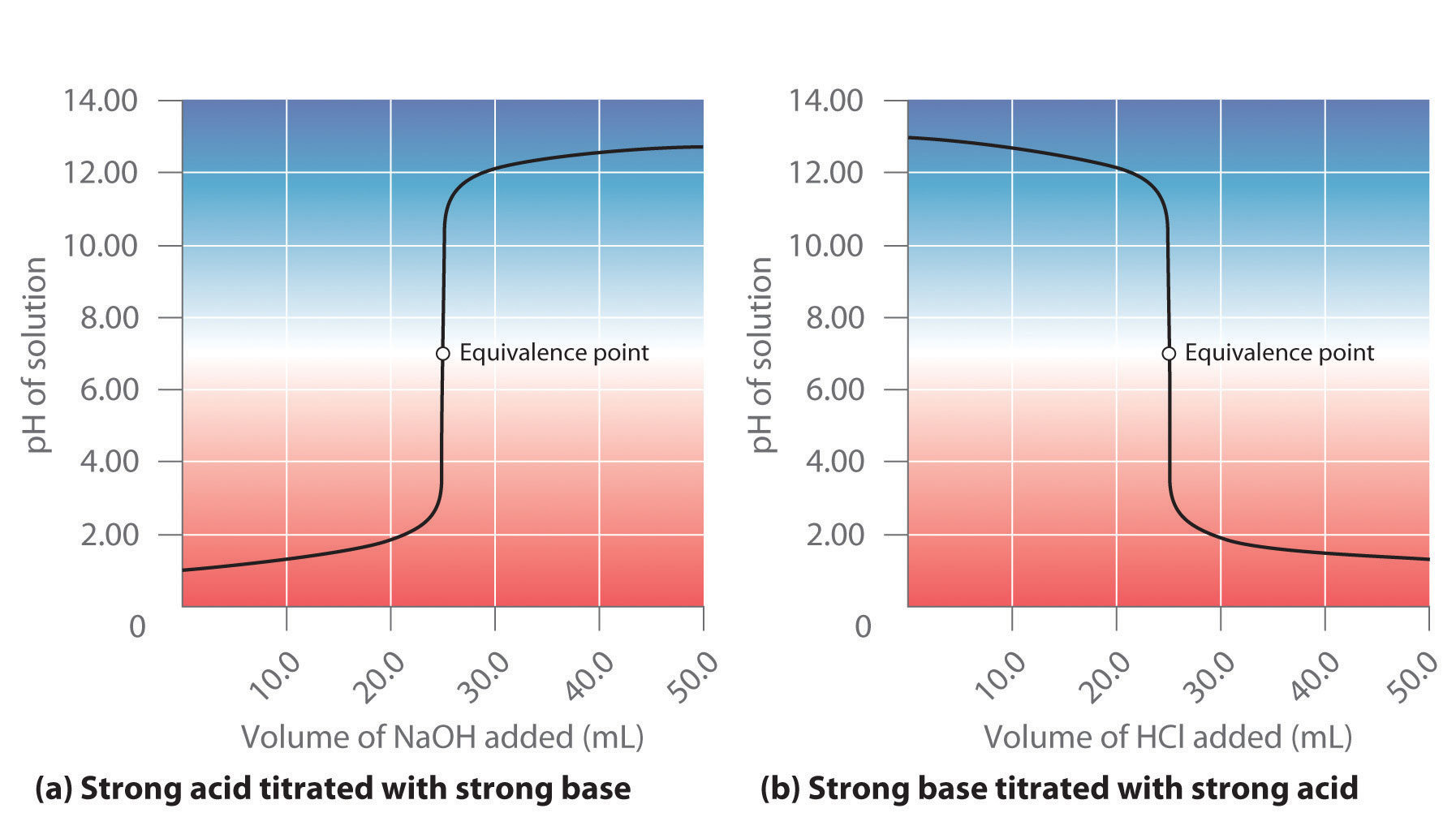
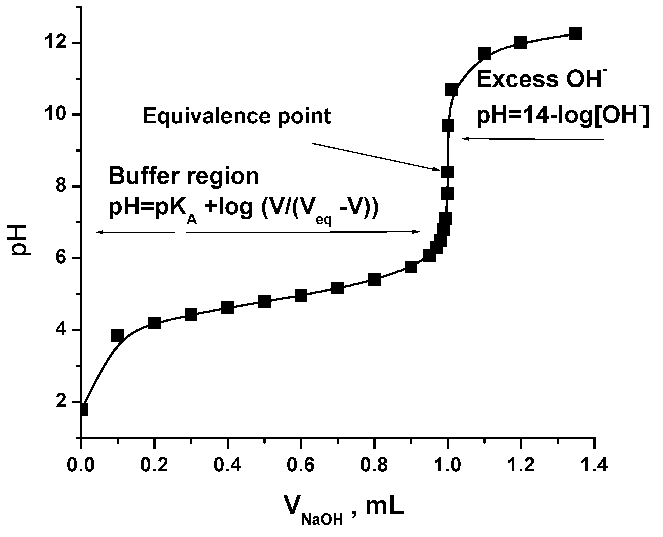
Here is an example of a titration curve, produced when a strong base is added to a strong acid. This curve shows how pH varies as 0.100 M NaOH is added to 50.0 mL of 0.100 M HCl.

Selective Focus of Sodium Hydroxide Base and Sulfuric Acid Solution in Brown Glass and Plastic Bottle Stock Photo - Image of base, hydroxide: 195465080







:max_bytes(150000):strip_icc()/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)

-in-water-01.jpg)