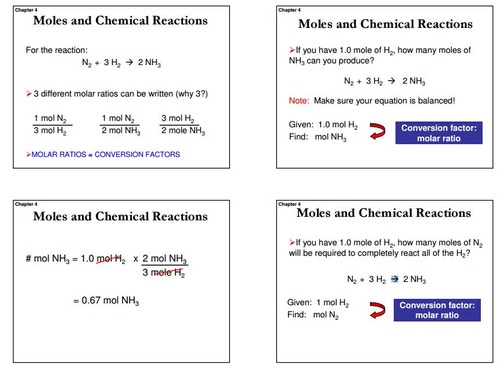
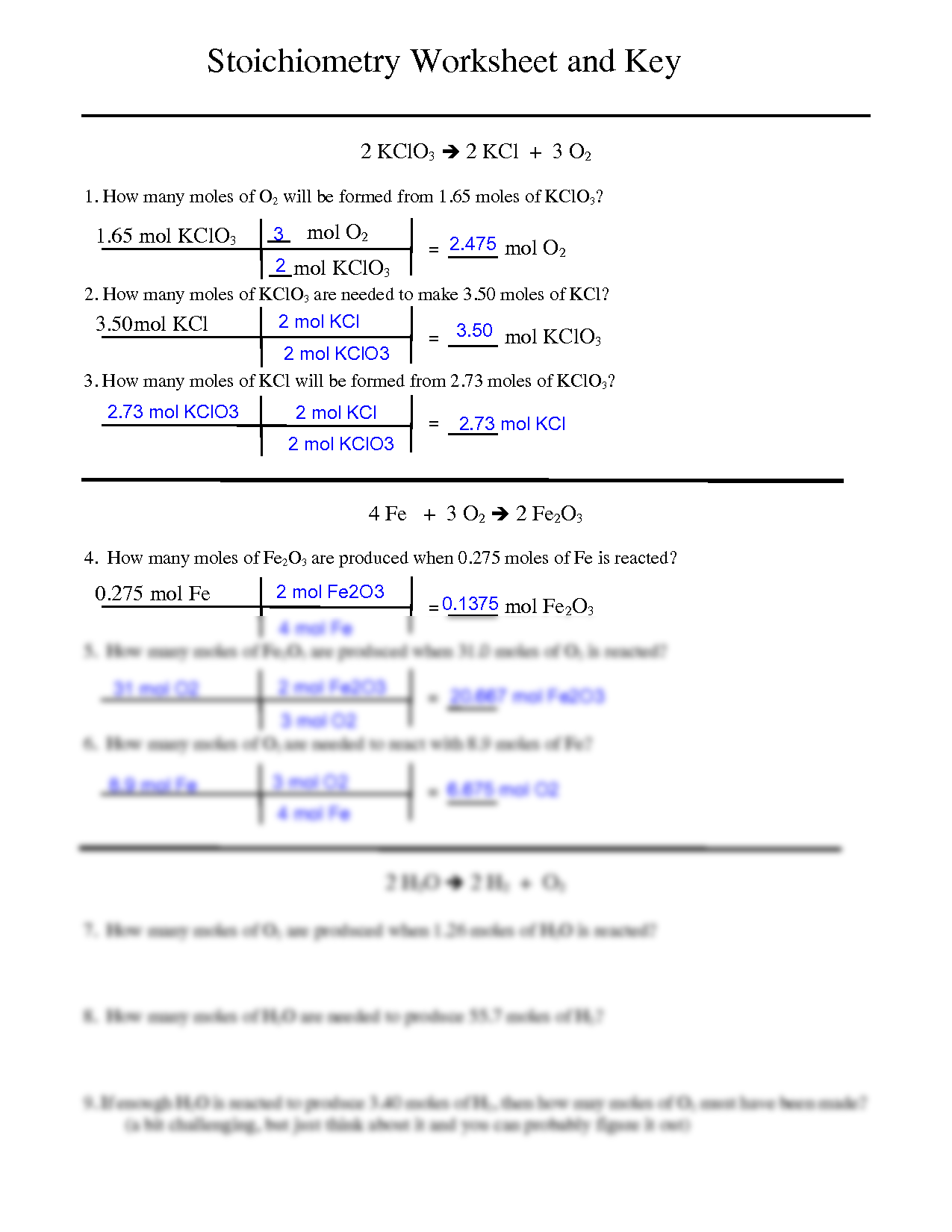
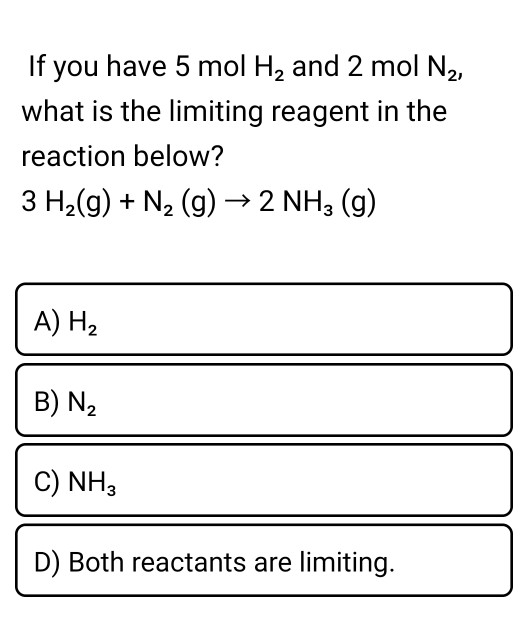
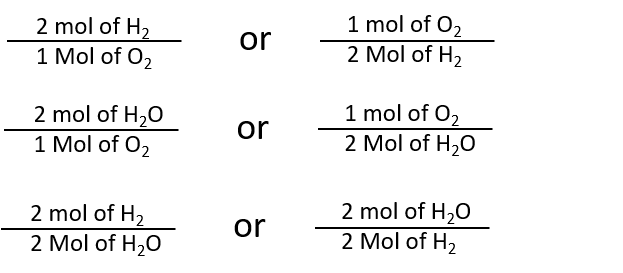
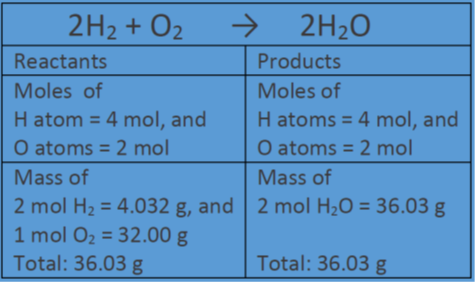
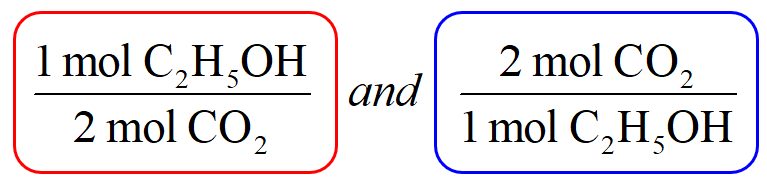32 The dissociation of CO2 can be expressed as 2CO2 converted into 2CO +O2. If 2 mol of CO2 is taken initially and 40
2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K - Sarthaks eConnect | Largest Online Education Community

How to Find Moles of Product from Moles of Reactant using a Chemical Equation | Chemistry | Study.com