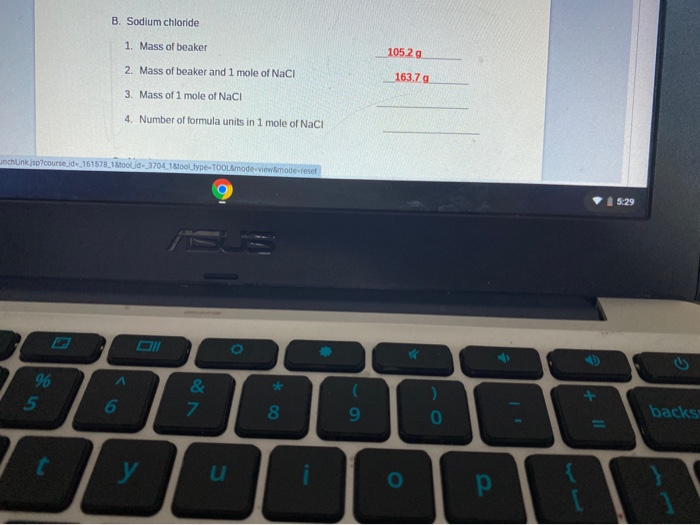
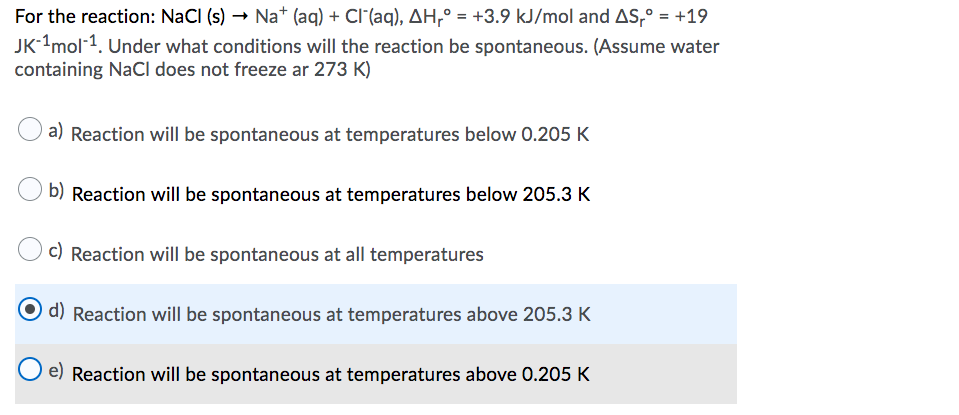
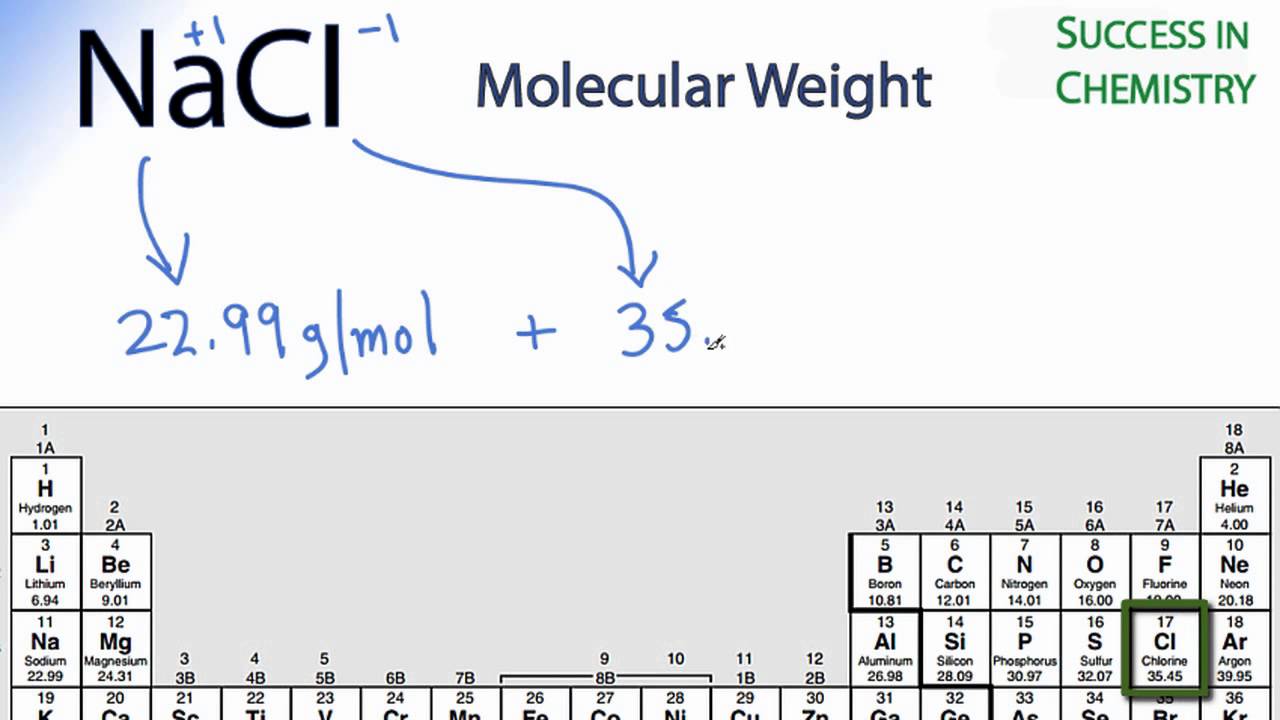
MOLE CALCULATIONS. Moles to Mass Find the mass of one mole of NaCl 58.5g Find the mass of two moles of NaCl 117.0g How did you get the answer? 2 moles. -

Static CA experiments at 1 mol/L NaCl and 45°C at muscovite surface.... | Download Scientific Diagram

How will the boiling point of a liter of water containing 1 mole of sodium chloride (NaCl) compare with that of a liter of water containing 1 mole of calcium chloride (CaCl2)?

Effects of 1 mol/L NaCl and the 1 mol/L NaNO3 electrolytes on surface... | Download Scientific Diagram

Calculate the mass of 1 mole of each one of the following: (a) `NaCl` , (b) `CaCO_(3)` , (c ) `FeSO - YouTube

XRD pattern of the sample immersed in the CaCl 2-NaCl-1mol% CaO melt... | Download Scientific Diagram

Explain why on addition of 1 mol of NaCl to 1 litre of water, the boiling point of water increases, while addition of 1 mol of methyl alcohol to one litre of
If the relative decrease in vapour pressure is 0.4 for a solution containing 1mol NaCl in 3 mol H2O,